Who Bcs Classification

- Bcs Classification Of Drugs
- Bcs Class 2 Drugs List
- Bcs Classification List
- Bcs Classification Of Drugs List
Dec 22, 2017 FDA has issued a final guidance entitled Waiver of In-vivo Bioavailability and Bioequivalence Studies for Immediate Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System. 37 BCS-based biowaivers are applicable to drug products where the drug substance exhibits high 38 solubility and, either high permeability (BCS Class I) or low permeability (BCS Class III). Jun 21, 2012 - The Biopharmaceutics Classification system (BCS) classifies drug substances based on aqueous solubility and intestinal permeability. Satisfy the criteria regarding solubility and permeability (BCS Class I and III), the drug. Product is an immediate-release oral dosage form with systemic action, and the drug product. Is a dosage form that is pharmaceutically equivalent to the reference product. In cases where.
Bcs classification system • 1. BIOPHARMACEUTICS CLASSIFICATION SYSTEM • Contents• Introduction• Overview of the Classification system• Applications• Conclusion• References • Introduction ۞Biopharmaceutics Classification System (BCS)♥ Scientific framework for classifying drug substances based on their aqueous solubility and intestinal permeability What is the need for a classification based on biopharmaceutics of the drug? Its importance in determining bioavailability • ORAL ROUTE♠ Route of choice for the formulators Continues to dominate the area of drug delivery technologies. LIMITATIONS Absorption and Bioavailability in the milieu of gastrointestinal tract. Limitations more prominent with the advent of protein and peptide drugs compounds emerging as a result of combinatorial chemistry and the technique of high throughput screening • API structure salt form and excipients Bioavailability of drug is determined byextent of drug solubility and permeability drug solubility drug product quality attributes • Biopharmaceutics Classification System Guidance provided by the U.S. Food and Drug Administration for predicting the intestinal drug absorption The fundamental basis established by Dr.
Gordon Amidon Distinguished Science Award (Aug ’06,FIP) First introduced into regulatory decision-making process in the guidance document on Immediate Release Solid Oral Dosage Forms: Scale Up And Post Approval Changes • Drug development tool that allows estimation of the contributions of 3 major factors, that affect oral drug absorption from immediate release solid oral dosage forms Dissolution Solubility Intestinal permeability.
Bcs Classification Of Drugs
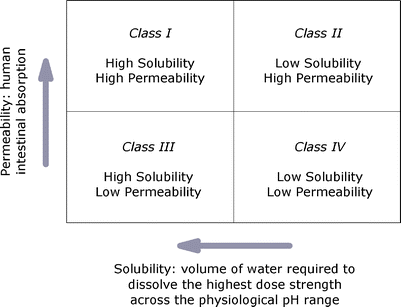
Biopharmaceutical Classification System and Formulation Development Particle Sciences - Technical Brief: 2011: Volume 9 The Biopharmaceutical Classification System (BCS) is an experimental model that measures permeability and solubility under prescribed conditions. The original purpose of the system was to aid in the regulation of post-approval changes and generics, providing approvals based solely on in vitro data when appropriate. Importantly, the system was designed around oral drug delivery since the majority of drugs are and remain orally dosed. Waivers, permission to skip in vivo bioequivalence studies, are reserved for drug products that meet certain requirements around solubility and permeability and that are also rapidly dissolving. More and more however, the industry is using the BCS as a tool in drug product development. As a simple example, BCS can be used to flag drugs that should not be tested clinically unless appropriate formulation strategies are employed (see Figure 1).
Bcs Class 2 Drugs List
Bcs Classification List
As an example, a BCS Class II compound, permeable but relatively insoluble, would likely not be a good clinical candidate without the use of enhanced formulation techniques aimed at increasing solubility or rate of dissolution. Various schemes exist that attempt to funnel a given API towards particular drug delivery techniques depending on the API’s BCS category.

Bcs Classification Of Drugs List
Still, most approaches remain fragmented in their methodology, ignoring commercially and biologically important factors. The BCS can however, when integrated with other information provide a tremendous tool for efficient drug development. One school of thought, very much endorsed by the authors, is that first in human (FIH) drug dosage forms should be designed to maximize bioavailability and that the FIH dosage form should be a logical step towards commercialization and not simply a stop gap to facilitate data acquisition. This makes sense both economically and ethically. For BCS Class I molecules, FIH formulations are straight forward and may consist of essentially the neat API. For other compounds, effective dosage forms present greater challenges. 3ds max 2014 activation code. Although designed originally to classify APIs as to their oral bioavailability, properly augmented, the BCS can be used as a key component of an algorithm to guide drug delivery system design for any route of administration.