Bsc Classification
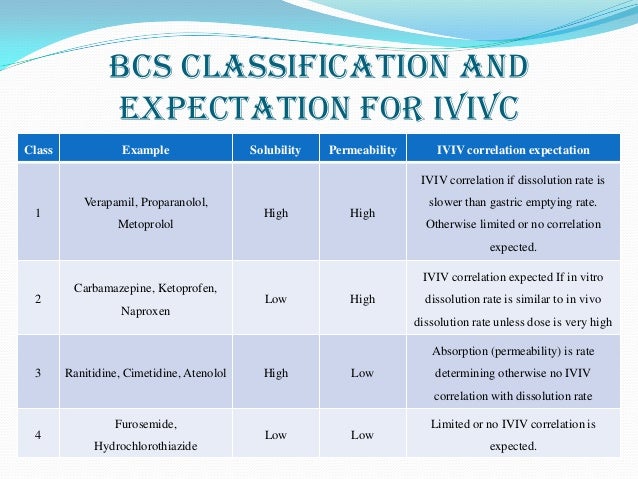
To provide sponsors of new drug submissions with the information necessary to comply with Division 8 of the Food and Drug Regulations ( Regulations) with respect to Biopharmaceutics Classification System (BCS) based biowaivers for comparative bioavailability studies to be used in support of the safety and efficacy of a drug. This information is applicable to all submission types where comparative bioavailability studies would normally provide pivotal evidence in support of the safety and efficacy of a product. In vivo human data collected for the purpose of submission to Health Canada should be collected in accordance with generally accepted clinical practices that are designed to ensure the protection of the rights, safety and well-being of subjects. They should be collected in compliance with the good clinical practices referred to in Division 5 of the Regulations and described in the International Conference on Harmonisation (ICH) Guidance (Topic E6) on Good Clinical Practice. The principles of Good Manufacturing Practice as indicated in Part C, Division 2 of the Regulations should be adhered to wherever applicable. Absorption - the uptake of substance from a solution into or across tissues.
Bcs Class 2 Drug
Absorption can include passive diffusion, facilitated passive diffusion (with a carrier molecule), and active transport. Critical dose drug - drugs where comparatively small differences in dose or concentration lead to dose- and concentration-dependent, serious therapeutic failures and/or serious adverse drug reactions which may be persistent, irreversible, slowly reversible, or life threatening, which could result in hospitalization or prolongation of existing hospitalization, persistent or significant disability or incapacity, or death. Adverse reactions that require significant medical intervention to prevent one of these outcomes are also considered to be serious. See Health Canada guidance document Comparative Bioavailability Standards: Formulations Used for Systemic Effects (22 May 2012) Dose solubility volume (DSV) - the highest therapeutic dose (milligrams) divided by the solubility of the substance [milligrams/millilitres (mg/mL)] at a given pH and temperature. For example, if a drug substance has a solubility of 31 mg/mL at pH 4.5 (37°C) and the highest dose is 500 mg, then DSV = 500 mg / 31 mg/mL = 16 mL at pH 4.5 (37°C). Highest dose - the highest approved therapeutic dose for the drug substance in Canada.

Restaurants Classification

Bcs Classification Ppt
If not currently approved in Canada, the highest proposed dose is applicable. Rapidly dissolving product - a product in which not less than 85% of the labelled amount is released within 30 minutes or less during a product dissolution test under the conditions specified in this guidance. Very rapidly dissolving product - not less than 85% of the labelled amount is released within 15 minutes or less during a product dissolution test under the conditions specified in this guidance. High solubility: A drug substance is classified as highly soluble if the highest therapeutic dose of the drug substance is completely soluble in 250 mL or less of aqueous media over the pH range of 1.2-6.8 at 37 ± 1°C, that is ( i.e.)., DSV less than or equal to (≤) 250 mL over the pH range. Low solubility: A drug substance is classified as a low solubility compound if the highest therapeutic dose of the drug substance is not completely soluble in 250 mL of aqueous media at any pH within the pH range of 1.2-6.8 at 37 ± 1°C, i.e., DSV greater than (>) 250 mL at any pH within the range. • Absolute bioavailability studies: Studies comparing the bioavailability of at least the highest dose of the drug substance from an orally administered aqueous solution, or an immediate-release tablet or capsule without excipients that are known to affect absorption, with an intravenous dose.